Abstract
Background: Hairy cell leukemia (HCL) is a rare lymphoproliferative disorder with an excellent prognosis following cladribine-based therapies. However, not all patients achieve complete remission and a significant proportion relapse after prior response. The addition of rituximab to cladribine deepens responses and whether this can prolong remission duration has not been established. Interestingly, some patients remain relapse-free despite having detectable residual hairy cells with long term follow-up.
Objective: Evaluate the correlation between peripheral blood (PB) and bone marrow (BM) measurable residual disease (MRD) assessment at one and three months into therapy and examine the role of PB flow cytometry (FCM) as an MRD tool during long term follow-up.
Methods: We retrospectively reviewed adult patients with HCL treated on a clinical trial with cladribine and rituximab at our institution between June 2004 and October 2020. Cladribine was administered at the dose of 5.6 mg/m2 daily for 5 days, and rituximab 375 mg/m2 weekly for 8 weeks, starting 28 days after the administration of cladribine. The correlation and concordance between FCM results obtained from BM and PB were evaluated using Pearson correlation and Bland Altman test, with linear regression performed to assess for proportional bias between the 2 techniques. Overall survival (OS) was calculated from the time of diagnosis to the time of death or last follow-up.
Results: 120 patients were analyzed, with a median age of 55 years (range, 29-91); 83% were males. Among 119 evaluable patients, 118 patients achieved complete remission (CR) and 1 patient CR with incomplete count recovery (CRi) as best response after a median of 81 days (range, 24-244). 101 patients were assessed for MRD by FCM on BM samples at best response, and 55% were found to be MRD negative.
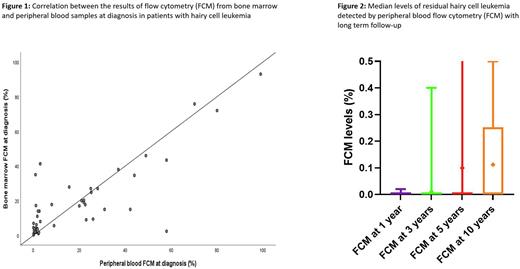
At diagnosis, the mean percentage of detectable hairy cells assessed by flow cytometry on BM and PB samples was 19% (range, 0.3%-93%) and 16% (range, 0%-99%) respectively. There was a good concordance between the two sample sources (R=0.82, P<0.0001) and no proportional bias (unstandardized coefficient=-0.16, P=0.10) (Figure 1). At 1 month, those respective levels were 1.4% (range, 0%-41%) and 1.8% (range, 0%-50%), with a strong correlation between sample sources (R=0.99, P<0.0001); however, proportional bias was observed (unstandardized coefficient=-0.30, P<0.0001). At 3 months, the median MRD level by BM was 0.27% (range, 0%-19%) and 0.14% by PB (range, 0%-6%), with good correlation between sample sources (R=0.98, P<0.0001).
From year 1 onwards after diagnosis, patients were monitored by FCM testing on PB for the evaluation of MRD (Figure 2). The mean MRD values at years 1, 3, 5, and 10 were 0.0003% (range, 0%-0.02%), 0.01% (range, 0%-0.4%), 0.10% (range, 0%-2.3%), and 0.11% (range, 0%-0.5%), respectively. Five patients relapsed: 4 patients with abnormal counts and 1 patient with BM showing relapsed disease in the setting of normal counts. None of the patients who relapsed had FCM at 1 year; one patient had positive MRD at 0.4% at 3 years and 0.25% at 5 years but relapsed at 8 years after diagnosis; and one patient had MRD at 0.15% at 10 years but his relapse manifested after 11 years from diagnosis. Two patients did not have any FCM measurements. Among the 115 patients who did not relapse, 78 had FCM tests at 1 year that were negative except for one positive result at 0.02% (became negative at 3 years), and 38 had FCM at 3 years with only two positive results at 0.01% (became negative at 5 years) and 0.03%. At 5 years, 24 patients had FCM assessment: one patient had a positive result at 0.03% (previously negative) and one at 2.3%. Four patients had FCM at 10 years and with no evidence of disease.
After a median follow-up of 101 months (range, 84-119), the median OS of the entire cohort was not reached, with a 10-year OS rate of 93%.
Conclusion: There is good concordance between FCM MRD assessed on BM and PB samples in patients with HCL particularly early in the course of therapy. Late relapses have been observed among patients in remission, justifying the use of FCM on PB as a reliable tool for long term MRD monitoring, with most relapsing patients having levels >0.1%.
Disclosures
Thompson:AbbVie, Gilead, Janssen, Pharmacyclics, Adaptive Biotechnologies, Genentech, Amgen: Honoraria; AbbVie, Gilead, Janssen, Pharmacyclics, Adaptive Biotechnologies, Genentech: Consultancy; AbbVie, Pharmacyclics, Adaptive Biotechnologies, Genentech: Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees. Kadia:Regeneron: Research Funding; Astex: Honoraria; Servier: Consultancy; Pfizer: Research Funding; Ascentage: Research Funding; Amgen: Research Funding; cyclacel: Research Funding; Delta-Fly: Research Funding; PinotBio: Consultancy; Iterion: Research Funding; Glycomimetics: Research Funding; Astellas: Research Funding; Genfleet: Research Funding; AstraZeneca: Research Funding; cellenkos: Research Funding; Novartis: Consultancy; JAZZ: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Agios: Consultancy; Abbvie: Consultancy, Research Funding. Alvarado:Jazz Pharmaceuticals: Research Funding; FibroGen: Research Funding; Sun Pharma: Research Funding; Astex Pharmaceuticals: Research Funding; Daiichi-Sankyo/Lilly: Research Funding; BerGenBio: Research Funding. Kantarjian:Jazz Pharmaceuticals: Research Funding; ImmunoGen: Research Funding; AbbVie: Honoraria, Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Novartis: Honoraria, Research Funding; NOVA Research: Honoraria; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Takeda: Honoraria. Ravandi:AstraZeneca: Consultancy; Abbvie: Consultancy, Honoraria, Research Funding; Xencor: Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Biomea Fusion, Inc.: Research Funding; Amgen: Honoraria, Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Prelude: Research Funding; Syos: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Amgen: Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.